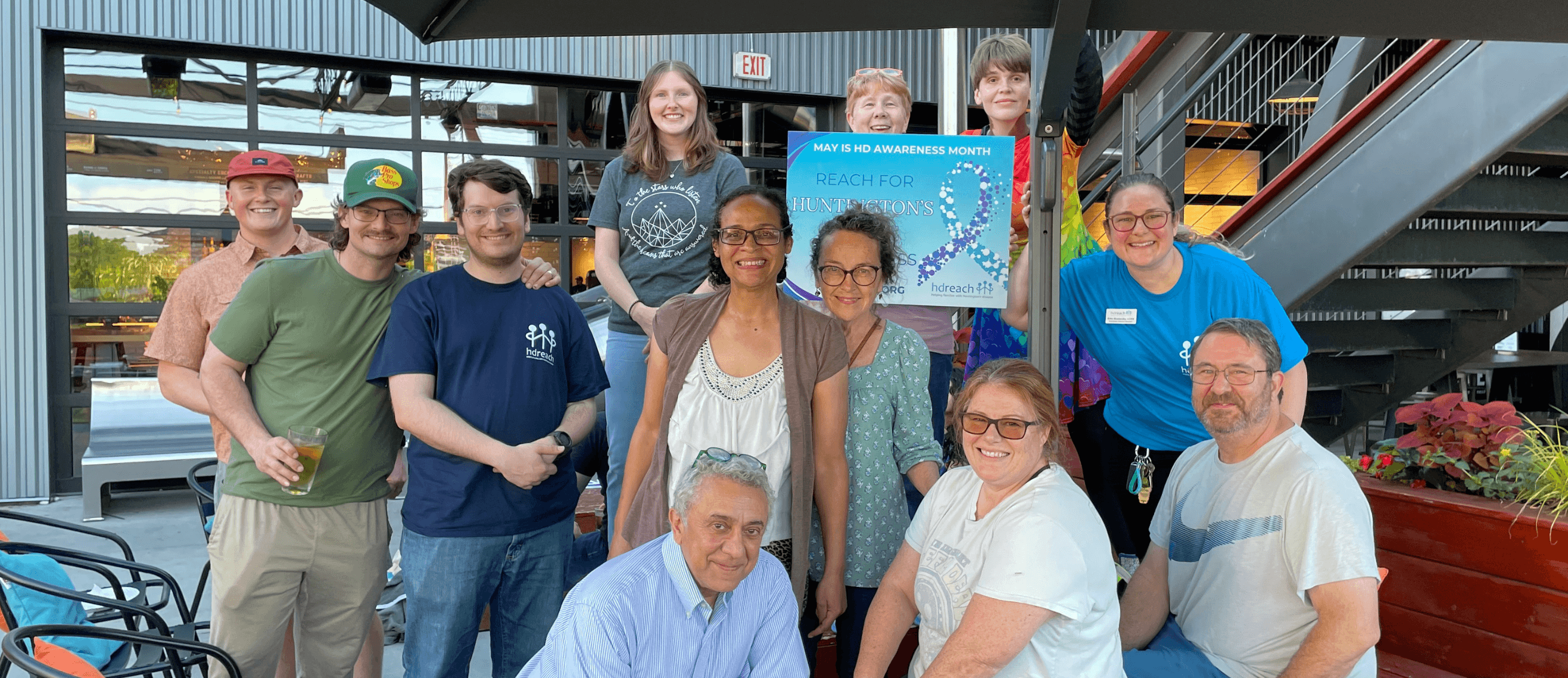
I had the honor of attending a meeting where organizations representing the Huntington’s disease community came together to deliver a petition to the FDA urging them to reverse the rollback on uniQure’s AMT-130 gene therapy application.
In September, uniQure announced that AMT-130 slowed the progression of HD by 75 percent over three years, a historic first for our community. For families who have only ever known decline, that news felt like hope we could finally hold onto. When the FDA unexpectedly shifted course on its agreed-upon review plan, advocates mobilized quickly, gathering more than 48,000 signatures from people who believe this therapy deserves a fair and timely path forward.
Although we were not permitted to enter the FDA building and deliver the petition in person that day, the HD community did what it always does. We found another way. The petition was delivered through other channels because this community is resilient. We do not stop when a door closes. We regroup. We adapt. We keep going.
I felt deeply honored to stand in that space, and I did not stand alone. I stood side by side with some of the strongest voices in our community. Leaders, caregivers, advocates, and families who have poured their hearts into this fight. Being there was not just meaningful. I take it as my responsibility to also be a voice.
As I looked around, I thought about every person I care about who is affected by HD in some way. Those living with it. Those at risk. Those who love someone with HD. And those we have lost too soon. I thought about my friends who are walking this journey every single day with courage and uncertainty.
The HD community is strong. We are resilient. We are relentless in our hope.
Thank you for allowing me to represent you. It was an honor I carry with deep gratitude.